IMPORTANCE OF A BROAD-SPECTRUM TOXIN BINDER FOR MAINTAINING HEALTHY GUT MICROBIOTA
Profitability of poultry farming depends up on faster growth of the birds, breast meat yield, efficiency of feed conversion rates and lower mortality and morbidity rates for broilers, and quality and quantity of egg production for layers. Plant based materials such as corn, soya, wheat bran, etc., are used as major ingredients for animal feed preparation. These ingredients are usually prone to toxigenic fungal attack, leading to contamination with single or multiple mycotoxins1. It has been proved by various studies that mycotoxins are having carcinogenic, estrogenic, immunotoxic, nephrotoxic, hepatotoxic and neurotoxic effects on animals and the effect is more pronounced, when multiple mycotoxins are present2,3,4. These effects can lead to a significant loss in profitability of poultry farming. Several researchers have investigated the mode of action of various mycotoxin and the pathways associated to pathogenesis1,4. In addition to this, some recent reports suggest that mycotoxins have a negative effect on beneficial gut microflora5.
Role of gut microbiota on health status of the animals
The gastrointestinal (GI) tract of chickens harbor diverse and complex microbiota that plays a vital role in digestion and absorption of nutrients, immune system development and pathogen exclusion6. In addition to this, the integrity, functionality, and health of the chicken gut depends on many factors including the environment, feed, and the GI microbiota. It has been reported that gut microbial diversity can be improved by probiotics6. Probiotics are single or mixed cultures of live non-pathogenic microorganisms, when provided in adequate amount confers benefit to the host1. Probiotics currently being used are Bacillus subtilis, lactic acid bacteria (LAB), i.e., (L. bulgaricus, L. acidophilus, L. casei, L. lactis, L. salivarius, L. plantarum), Streptococcus thermophilus, Enterococcus faecium, E. faecalis, Bifidobacterium spp., fungi (Aspergillus oryzae) and yeast (Saccharomyces cerevisiae)7.Teo et al., have reported that broiler birds fed with B. subtilis (PB6) had heavier bursas and heterophils with higher in vitro phagoctyosis for Escherichia coli8. This indicates the role of B. subtilis (PB6) on chicken innate immune system possibly by supporting gut microflora8. Such findings indicate that the interactions between animal and gut microbiota is fundamental to poultry health and production6,9.
Interaction between mycotoxin and the gut microbiota: Bi-directional effect
Alteration in the GI gut microbiota is caused by non-infectious and infectious stressors6. The non-infectious factors include environmental stressors, nutritional imbalances, dietary changes, mycotoxins, poor management, enzymatic dysfunction, or host genetics6. Infectious factors include viral or bacterial challenge, coccidiosis, or toxic metabolites produced by harmful microorganisms such as Clostridium perfringens. Mycotoxins being a non-infectious stressor, have been demonstrated for modulation of gut microbiota composition6. Such alteration in gut microbiota can be observed up to species level in some of the studies. In another study, it was found that each mycotoxin has differential growth inhibiting effect on bacteria on exposure to mycotoxins (Table 1)10. In a study reported by Ali-Vehmas et al., mycotoxins slowed down the growth of microorganisms indicating the possibility of antimicrobial effect11.
|
Reference: Madhyasthal, et al. 1994 |
Table 1shows the microbial sensitivity of mycotoxins.
|
Mycotoxin |
Microbes shown sensitivity |
Microbes shown insensitivity |
|
Aflatoxin B1 |
Bacillus subtilis, B. thuringiensis, B. pumilus, B. brevis, and B. cereus |
Steptococcus aureus, E. coli, Salmonella typhimurium and Saccharomyces cerevisiae |
|
Ochratoxin A |
B. brevis, B. cereus |
Bacillus subtilis, B. thuringiensis, B. pumilus, Saccharomyces cerevisiae and E. coli |
|
Cyclopiazonic acid |
Bacillus subtilis, B. thuringiensis, B. pumilus, B. brevis, and B. cereus |
Steptococcus aureus, E. coli, Salmonella typhimurium, Saccharomyces cerevisiae and E. coli |
|
Zearalenone |
B. brevis, B. cereus and Bacillus subtilis |
B. thuringiensis, B. pumilus and E. coli |
|
T-2 |
Saccharomyces cerevisiae |
Bacillus subtilis, B. thuringiensis B. pumilus, B. brevis, B. cereus and E.coli |
Beneficial bacteria such as Bacillus subtilis and B. pumilis was found to be sensitive to aflatoxin B1 (AfB1), cyclopiazonic acid (CPA) and zearalenone (zea), whereas T-2 was sensitive to Saccharomyces cerevisiae. Ochratoxin A (OTA) was sensitive to B. brevis and B.cereus. None of the tested mycotoxins was reported to be sensitive to E. coli and Salmonella10. Though mycotoxins shown sensitivity to the microbes, the extent of sensitivity depends on the concentration of mycotoxins and the environment it is exposed to. The reported effects of most of the mycotoxins are negative in terms of intestinal health accompanied by an increase of the gut pathogen2. Fumonisins are reported to have the ability to modify microbiota by modulation of mucus production5. In addition to this, alteration in composition of intestinal microbiota of ileum was also reported in broiler birds administered with FB for 15 days5. The alteration was primarily seen in reduction of beneficial bacteria such as Candidatus savagella and Lactobacillus. In contrary to this, microbes attack mycotoxins by either adsorption or biotransformation mode also and thus mitigates the toxic effects of mycotoxins2,5. Reports on gut microbiota eliminating mycotoxin from the host naturally also exists, provided that the host is healthy with a balance gut microbiota6. Such findings convey that, mycotoxins and microbes have bi-directional effect.
Occurrence of mycotoxins
United Nations Food and Agriculture Organization and the World Health Organization has estimated that 25% of the world’s crops such as nuts, cereals, and rice are contaminated by mold and fungal growth2. Mycotoxins are exposed mostly by ingestion followed by the dermal and inhalation routes2. Mycotoxin exposure is not only limited to pure mycotoxins, but also masked mycotoxin which are formed by conjugating mycotoxins to biopolymers2. The extent of adverse effects of mycotoxins on animals’ health mainly depends on the extent of exposure (dosage and period), type of mycotoxins, physiological and nutritional status as well as possible synergistic effects of other chemicals to which the animals are exposed2. Around 400 mycotoxins are known, but AFs, ochratoxins, zearalenone (ZEA), fumonisins (FBs) and trichothecenes are mostly focused. The emerging mycotoxins, CPA and MPA are also seen in the animal feed which can substantial loss in the farming3.
Mycotoxins level in the animal feed
In an analysis in animal feed done by Kemin customer laboratory services, it was found that at least 85% of the samples was found to be contaminated with either single or multiple mycotoxins12. Mycotoxin contamination in the samples beyond the safe limits recommended by European Food Safety Authority (EFSA) varied from 5% to 45% depending upon the mycotoxins, amongst AfB1 dominated. It was also observed that, mycotoxin/s below safer limits are also present in most of the animal feed samples4. Such difference in low to high contamination level of mycotoxins varied according to the climatic season as well4.
In another published report, AfB1 and T-2 found to be mostly prevalent with 42% and 40% above risk tolerance level, respectively13. The same report shows 75% of the samples was contaminated with OTA, of which, 8% sample was found to be above the tolerance level12,13. Contamination with fumB1 and T-2 was found to be 17% and 57% respectively. Multi mycotoxin contamination is also reported and it is a big concern because of the synergistic toxic effects11. The report shows 95% of the tested sample was contaminated with mycotoxin. In the animal feed to the extent of 17.22 %, 30%, 35% and 13% was found to be contaminated with a single mycotoxin, two mycotoxins, three mycotoxins and four mycotoxins, respectively13.
Does the contamination level decide the selection or addition of toxin binder?
Mycotoxins analysis in the feed reveals that animal feed is contaminated with broad spectrum of mycotoxins such as AfB1, OTA, fumonisin B1 (fum B1), zea, T-2, CPA and DON in the animal feed12,14. There are instances where the contamination levels in the animal feed are seen below and above the tolerance levels suggested by European Feed Safety Authority (EFSA). During high level of contamination, broad spectrum toxin binders are used, whereas in less contamination level (below EFSA limits), broad spectrum efficacious toxin binders or sometimes toxin binders are not used. From the published reports it is obvious that, mycotoxins have negative effect on beneficial microbiota and on animal itself (synergistic effect of mycotoxins). This suggests that, mycotoxins below the EFSA safer limits can affect the animal and hence broad-spectrum toxin binder/s, needs to be used in the animal feed without exception.
Need of broad-spectrum toxin binder
Considering that most feed samples are contaminated with multiple mycotoxins, a broad-spectrum mycotoxin binder is required for eliminating the risk. An ideal mycotoxin binder for animal feed application should have several qualities. First and foremost, it should have high binding efficacy against all common mycotoxins. It needs to be noted that poultry GI system has different pH at different locations and the binding should be intact when the feed passes through the GI system. Secondly, it should not bind to essential nutrients present in the feed such as vitamins and minerals. This is very important as materials such as charcoal show high binding efficacy against nutrients. It is also important to note that the mycotoxin binder should not get digested/degraded while passing through the GI tract as it can lead to release of the bound mycotoxins.
TOXFIN™ 300:
Kemin Agrifoods India launched Toxfin 300, a broad-spectrum toxin binder composed of hybrid nano-silicates, activated clays and organic acids. Toxfin 300 was developed and tested using biphasic in vitro assay in context to poultry with mycotoxins and vitamins. It was then tested in vivo conditions with broiler birds using mycotoxin excretion method. The biphasic in vitro assay involves adsorption at pH 3.0 simulating the pH of crop and desorption at pH 6.8, simulating the pH of intestinal condition. Based on adsorption and desorption, net binding was calculated
Mycotoxin net binding efficiency (%) = Adsorption at pH 3.2 (Upper intestinal pH, acidic condition) – desorption at pH 6.8 (Lower intestinal pH, neutral to slightly alkaline condition)
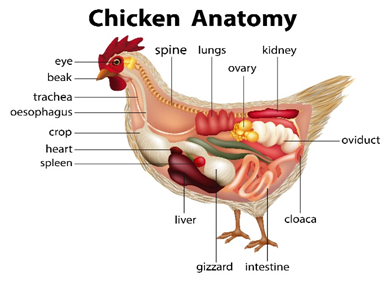
Figure 1 shows the anatomy of the chicken and its relevance for the in vitro method
The binding efficacy of Toxfin 300 and other products having different actives towards mycotoxins are shown in Figure 214. The result shows, Toxfin 300 containing HNS and activated clay as key ingredient performed much better than the products having different actives. It was also observed that product having HSCAS as active showed good binding to AfB1 alone. Though products having other actives showed binding to most of the mycotoxins, but higher binding was observed with Toxfin 300.

Figure 2. In vitro binding efficacy of mycotoxins for the products. Each experimental data represents mean net binding +/- standard error (n=3). Afla B1 – Aflatoxin B1, OTA – Ochratoxin A, ZEA – Zearalenone, CPA – Cyclopiazonic acid, MPA – Mycophenolic acid, Fum B1 – Fumonisin B1. *Analysis not done; ** - Data represents adsorption at pH 3.2;
Toxfin 300 was then tested with vitamin B6 using the same procedure along with products of different actives and the results are shown in Figure 314. Among the tested products, Toxfin 300 showed the least binding. It was also observed that clay-based product exhibited high binding to vitamin B6.

Figure 3. In vitro net binding efficacy of products towards vitamin B6. Each experimental data represents mean net binding +/- standard error (n=3).
Toxfin 300 binding efficacy towards mycotoxins was also confirmed using in vivo excretion study14. In vivo excretion study consists of administering the feed contaminated with known amount of mycotoxins (OTA-100 ppb, zea-100 ppb and AfB1-50 ppb) admixed with the products of different actives after starving the broiler bird. Then the excreta were collected, pooled, dried and segregated according to the group receiving the respective actives and the results are shown in Figure 4. Toxfin 300 group showed higher excretion of all the mycotoxins among the tested products. All the products showed similar excretion efficacy for AfB1, whereas for OTA and zea, Toxfin 300 outperformed.

Figure 4. In vivo mycotoxin binding study with broiler birds. Data shows the relative percentage excretion of mycotoxins with respect to Toxfin 300 (mean, n=12). Superscripts with different alphabets denotes statistical difference between the products (p<0.05).
Conclusion:
In the current scenario, animal feed is contaminated with multiple mycotoxins ranging from low to high levels. Mycotoxins has growth inhibiting effect on beneficial bacteria thereby alter the gut microbiota, which plays a major role in maintaining the intestinal health and overall health of the bird. So, it is essential to use a broad spectrum, efficacious toxin binder in the animal feed, even at low level of mycotoxin contamination to prevent the alteration of gut microbiota by mycotoxins.
Source: KEMIN